Electrolytes
Electrolytes
Organic electrolytes play a critical role to achieve high voltage and a longer lifespan for rechargeable batteries. We develop new electrolytes and additives to meet the challenges of graphite passivation, sulfur chemistry, as well as low temperature environment.
Projects
Featured Publications
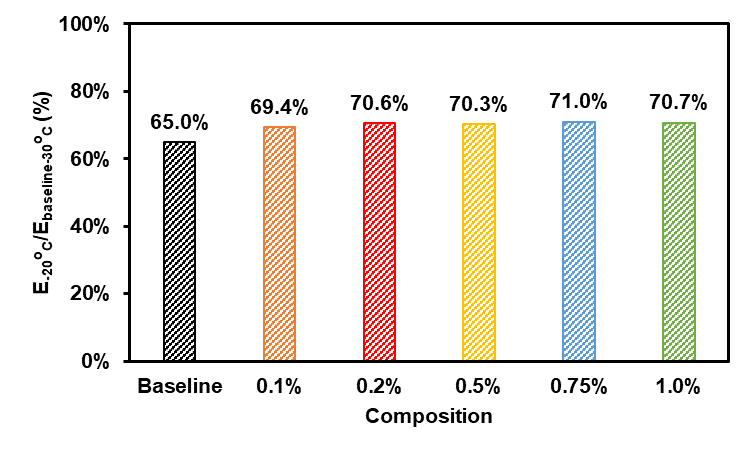
Hubble, Dion, David Emory Brown, Yangzhi Zhao, Chen Fang, Jonathan Lau, Bryan D McCloskey, and Gao Liu."Liquid electrolyte development for low-temperature lithium-ion batteries."Energy & Environmental Science
15.2 (2022) 550 - 578. DOI
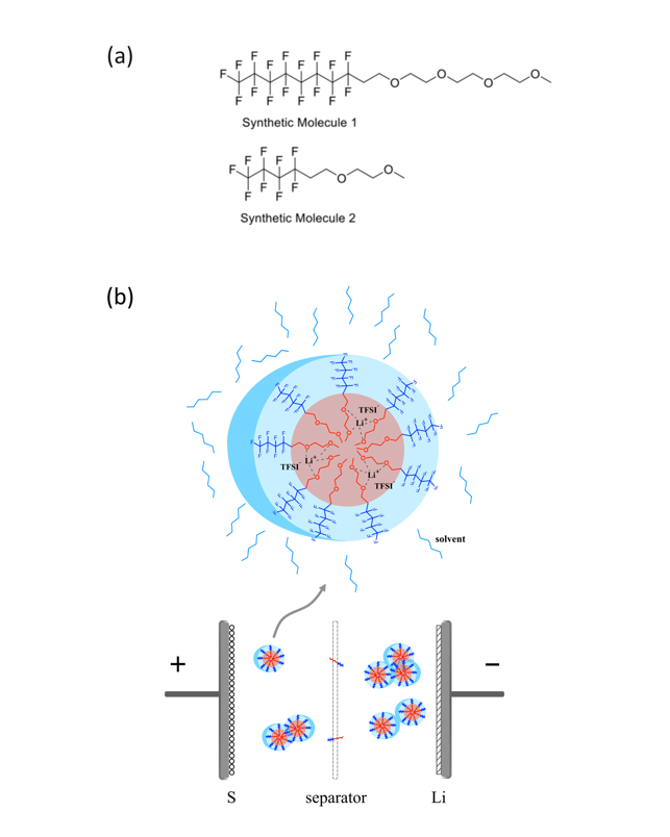
Zhao, Yangzhi, Chen Fang, Guangzhao Zhang, Dion Hubble, Asritha Nallapaneni, Chenhui Zhu, Zhuowen Zhao, Zhimeng Liu, Jonathan Lau, Yanbao Fu, and Gao Liu."A Micelle Electrolyte Enabled by Fluorinated Ether Additives for Polysulfide Suppression and Li Metal Stabilization in Li-S Battery."Frontiers in Chemistry
8 (2020). DOI
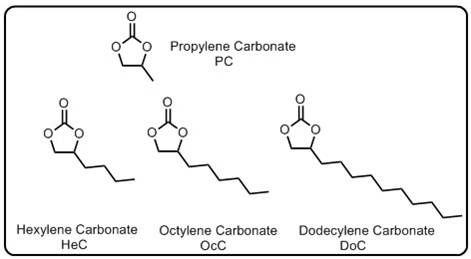
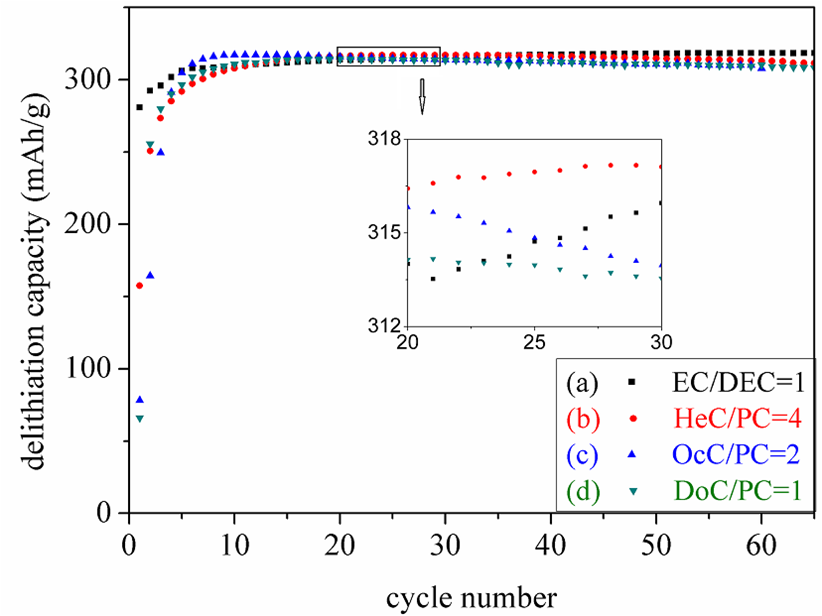
Zhao, Hui, Sang-Jae Park, Feifei Shi, Yanbao Fu, Vincent S Battaglia, Philip N Ross, and Gao Liu."Propylene Carbonate (PC)-Based Electrolytes with High Coulombic Efficiency for Lithium-Ion Batteries."Journal of The Electrochemical Society
161.1 (2014) A194-A200. DOI