Lithium-Sulfur Batteries
Lithium-Sulfur Batteries
Lithium sulfur rechargeable battery is potentially low cost and high energy storage chemistry, because sulfur is an abundant element, and can be mined at low cost. However, LiS chemistry has many challenges due to the polysulfides dissolution, and inhomogeneous lithium metal deposition during charge and discharge process. We aim to address these challenges through design, synthesis and formulation of new electrolytes, and electrode engineering.
Projects
Featured Publications
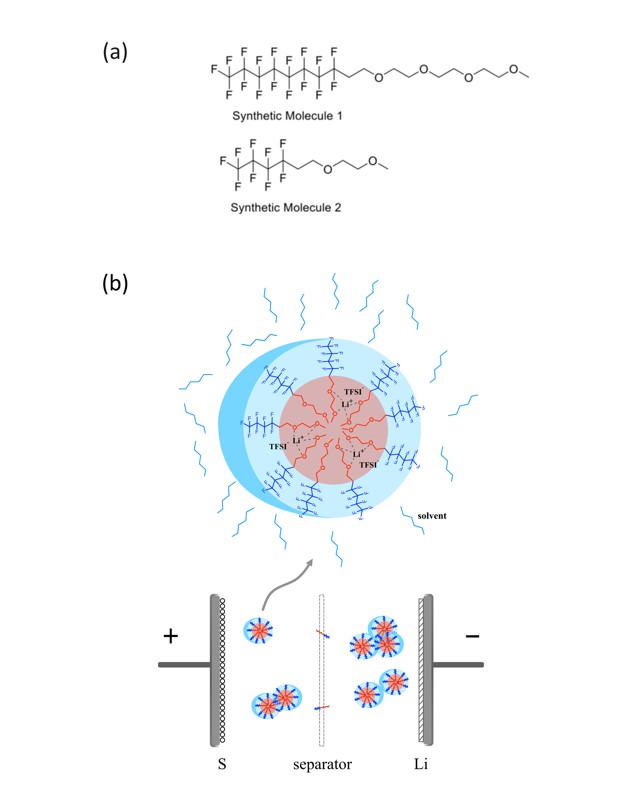
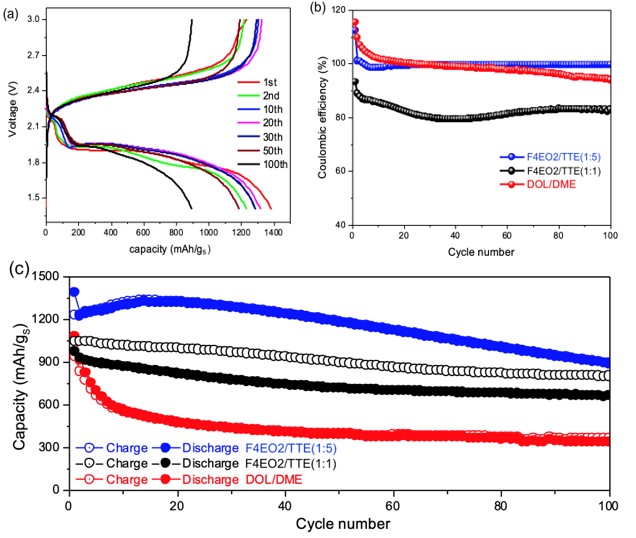
Zhao, Yangzhi, Chen Fang, Guangzhao Zhang, Dion Hubble, Asritha Nallapaneni, Chenhui Zhu, Zhuowen Zhao, Zhimeng Liu, Jonathan Lau, Yanbao Fu, and Gao Liu."A Micelle Electrolyte Enabled by Fluorinated Ether Additives for Polysulfide Suppression and Li Metal Stabilization in Li-S Battery."Frontiers in Chemistry
8 (2020). DOI
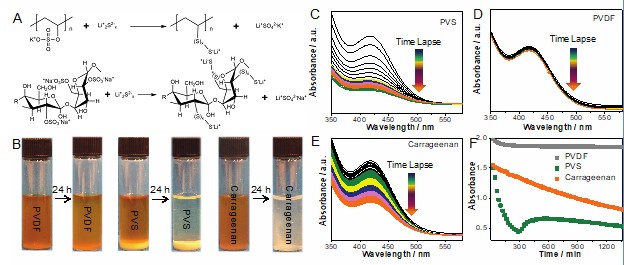
Ling, Min, Liang Zhang, Tianyue Zheng, Jun Feng, Jinghua Guo, Liqiang Mai, and Gao Liu."Nucleophilic substitution between polysulfides and binders unexpectedly stabilizing lithium sulfur battery."Nano Energy
38 (2017) 82 - 90. DOI
Ai, Guo, Zhihui Wang, Yiling Dai, Wenfeng Mao, Hui Zhao, Yanbao Fu, Yunfei En, Vincent S Battaglia, and Gao Liu."Improving the over-all performance of Li-S batteries via electrolyte optimization with consideration of loading condition."Electrochimica Acta
218 (2016) 1 - 7. DOI
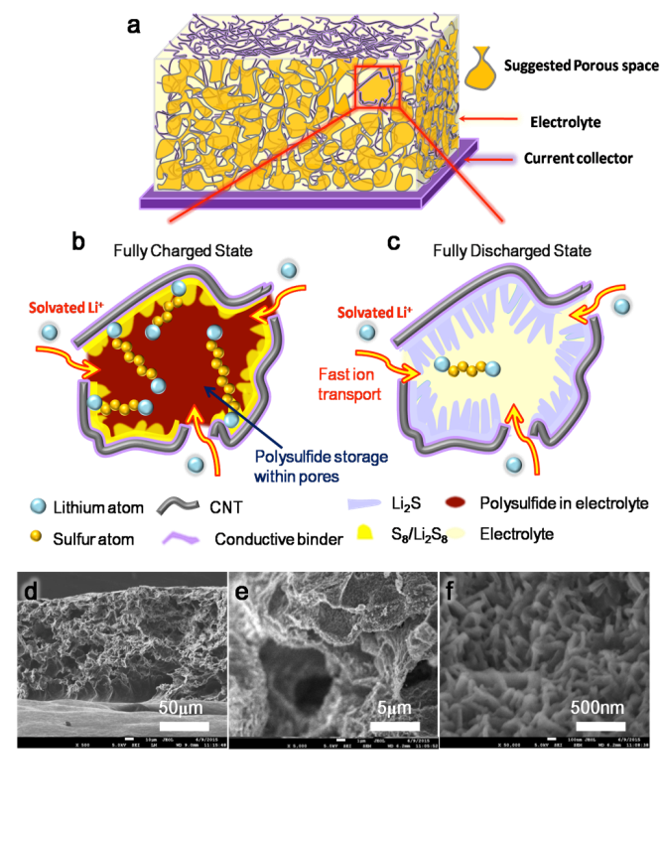
Ai, Guo, Yiling Dai, Wenfeng Mao, Hui Zhao, Yanbao Fu, Xiangyun Song, Yunfei En, Vincent S Battaglia, Venkat Srinivasan, and Gao Liu."Biomimetic Ant-Nest Electrode Structures for High Sulfur Ratio Lithium–Sulfur Batteries."Nano Letters
16.9 (2016) 5365 - 5372. DOI